Australia’s commitment to safeguarding free speech is deeply rooted in its constitution and human rights laws. However, this fundamental right is not absolute, as reasonable limitations exist, such as restrictions on hate speech. The advent of the internet and social media has amplified the reach and impact of free expression, granting individuals unprecedented access to information and enabling political mobilization on an unprecedented scale.
However, this technological revolution has also ushered in new challenges that test the boundaries of free speech. In recent months, Australia has found itself at the center of an international discourse due to a high-profile clash between Elon Musk’s social media platform, X (formerly Twitter), and the Australian government. The controversy arose over the circulation of videos depicting a church stabbing incident, underscoring the intricate relationship between free speech and technology in the modern era.
Musk’s stance in this situation can be seen as an attempt to uphold free speech against perceived attempts by the Australian government to exert pressure on X and other social media companies to impose restrictions on the dissemination of such content. This clash highlights the delicate balance that must be struck between upholding the fundamental right to free expression and addressing legitimate concerns over the potential harm caused by the unrestrained spread of certain types of content.
Notably, the debate around free speech online has extended beyond this specific incident, permeating into various sectors, including healthcare and cosmetics. As we navigate the complexities of this digital age, it becomes increasingly crucial to engage in thoughtful discourse and find a balanced approach that respects fundamental rights while mitigating potential risks to public safety and well-being.
The Ethical Responsibility of Healthcare Professionals
As the free speech debate extends into various sectors, the healthcare industry finds itself grappling with the ethical implications of online expression by medical professionals. Striking the right balance between free speech and maintaining public trust becomes crucial. In the ongoing digital age, the intersection of free speech, technology, and public health has emerged as a complex and nuanced issue. On one hand, the internet and social media platforms have provided an unprecedented platform for individuals to express their opinions and engage in public discourse. On the other hand, the spread of misinformation and harmful content on these platforms has the potential to undermine public health efforts and endanger the well-being of individuals and communities.
As a result, finding a balance between protecting free speech rights and safeguarding public health has become a pressing challenge for policymakers, ethicists, and technology experts alike. This challenge is further amplified by the rapid evolution of technology and the increasing influence of social media platforms in shaping public opinion. Consequently, regulatory bodies have taken steps to address these concerns, particularly in the realm of healthcare and cosmetic services.
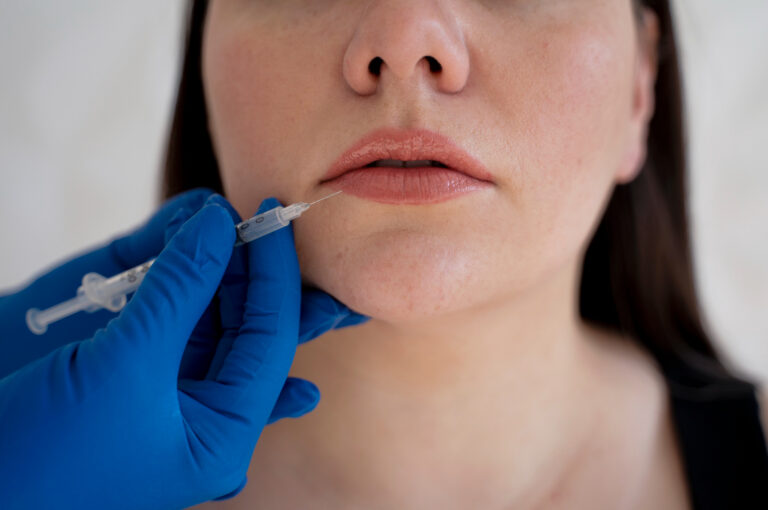
The Debate Around Cosmetic Surgery Advertising: A Battle of Semantics
In response to these challenges, the new restrictions on cosmetic surgery advertising in Australia, enforced as of July 1, 2023, were implemented by the Medical Board of Australia and the Australian Health Practitioner Regulation Agency (AHPRA). These new guidelines aim to address concerns around the lack of clarity on practitioner qualifications, the elective nature of cosmetic surgery driving demand through advertising, and the use of potentially problematic social media tactics like influencer promotion. These guidelines prohibit the use of testimonials, require patient consent for images, mandate full risk disclosure, and ban depiction of unrealistic expectations in ads.
While currently limited to invasive surgical procedures like breast augmentations and facelifts, indications suggest similar restrictions may extend to non-surgical treatments like Botox (onabotulinumtoxinA) and dermal fillers.
Additionally, the Therapeutic Goods Administration (TGA), responsible for overseeing the advertising of therapeutic goods, has implemented stricter regulations for promoting cosmetic injectables. This shift comes in response to concerns about potentially misleading terminology used in advertisements.
In late 2023, the Therapeutic Goods Administration (TGA) in Australia introduced new guidelines restricting the advertising of cosmetic injectable treatments containing prescription-only medicines. The widely used phrases “anti-wrinkle injections” and “dermal fillers” were among the terms that could no longer be used, as they were deemed to promote specific prescription medicines.
Effective January 2024, the TGA has implemented a comprehensive ban on the use of terms like “anti-wrinkle injections”, “wrinkle-reducing injections”, or “dermal fillers” in advertisements for cosmetic injectable treatments involving prescription-only substances. This ban extends beyond just the explicit terms, encompassing any direct or indirect references that could be associated with Schedule 4 prescription medicines commonly used in cosmetic injectable procedures. This includes the use of acronyms, nicknames, abbreviations, and even hashtags that may be linked to specific prescription-only substances found in these treatments.
The intent behind this stringent measure is to prevent any advertising that could be perceived as endorsing or promoting the use of particular prescription medications, which falls outside the scope of permitted advertising practices for therapeutic goods in Australia.
The rationale behind this move is twofold: firstly, to prevent the creation of inappropriate consumer demand for prescription medicines, and secondly, to safeguard the health professional-patient relationship by avoiding advertising that could be misconstrued as endorsing particular prescription-only products.
Under the new guidelines, clinics and practitioners are required to revise their advertising and marketing materials, removing any prohibited terms and avoiding references to specific prescription-only products used in cosmetic injectable treatments. Instead, they can advertise their services more generally, such as “Our clinic can provide consultations on reducing the appearance of wrinkles”, without specifying the products used.
The TGA’s new guidelines aim to align the cosmetic injectables industry with ethical advertising standards for healthcare services. While raising initial concerns about consumer confusion and costs of revising materials, the stricter regulations ultimately benefit consumers and responsible businesses. By prohibiting terms that promote specific prescription medicines, the focus shifts to clear, truthful advertising. Ethical clinics prioritizing patient education and informed consent will likely adapt smoothly. Overall, the emphasis on transparent, responsible marketing enhances consumer trust and levels the playing field for businesses upholding safety and ethical practices in this industry.
Another significant challenge arises from unregulated overseas cosmetic providers, often based in Turkey, South America, and Southeast Asia, operating outside local regulations. This lack of oversight allows them to advertise freely, potentially risking consumer safety and undermining Australian regulators’ efforts to protect public health and industry standards.
Enforcement Challenges and Regulatory Gaps
While AHPRA (Australian Health Practitioner Regulation Agency) has acknowledged receiving around 200 notifications regarding non-compliant advertising practices in the past, there is a lack of clear, evidence-based data demonstrating the effectiveness of the measures taken to address these violations. This absence of tangible proof raises questions about the real impact of the agency’s efforts in curbing non-compliant advertising within the cosmetic industry.
Moreover, AHPRA’s regulatory focus is primarily directed towards registered healthcare practitioners, creating a substantial gap in addressing fraudulent businesses operated by individuals without healthcare qualifications. This limitation in the agency’s scope of authority creates a potential loophole that unscrupulous entities may exploit to circumvent the advertising regulations.
Both AHPRA and the Therapeutic Goods Administration (TGA) have claimed to be pursuing legal actions against violators of the advertising guidelines. However, the burden of proof required for such legal proceedings is often higher, and the implementation of enforcement measures faces significant challenges. Consequently, despite the regulatory bodies’ efforts, cosmetic practitioners who comply with the rules frequently bear a disproportionate burden of these regulations, while those operating outside the legal framework may continue to flout the guidelines with relative ease and perhaps impunity.
Navigating Competing Interests
These recent regulations on advertising cosmetic injectables and surgery have sparked debate, impacting businesses like Norwest Cosmetic Surgery, marketers, and individuals seeking information about these procedures. Proponents argue that the restrictions are necessary to protect consumers from potentially misleading or harmful advertising practices. However, critics contend that the regulations unduly limit companies’ ability to communicate with their target audience and restrict consumers’ access to information about available products and services.
The heart of this debate lies in a fundamental tension: shielding consumers from deceptive advertising for medical and cosmetic services, while ensuring their access to truthful information about these options. This is especially important because safeguarding public safety is a legitimate concern. However, careful consideration is needed for regulations that do not infringe upon the fundamental principles of free expression and access to information.
This intersection of legal and ethical considerations in medical practices and free speech underscores the complexities of maintaining a democratic society that values both individual liberties and public well-being. Navigating these intricate issues requires thoughtful dialogue and a collaborative exploration of solutions that uphold the principles of free speech while simultaneously addressing legitimate public health concerns.
Ultimately, the cosmetic advertising ban has ignited a broader conversation about the role of regulation in the beauty industry and the need to strike a balance between protecting consumers and preserving the rights of businesses and individuals to engage in commercial communication and access information freely.
The Question of Fairness
There have been concerns about the ban’s potential lack of fairness. Cases of AHPRA investigating cosmetic practitioners for social media posts highlight this tension. While details remain confidential, they demonstrate AHPRA’s commitment to holding professionals accountable for online conduct that might erode public trust. However, these measures primarily target registered health practitioners, leaving a gap in addressing fraudulent businesses owned by non-healthcare practitioners. While AHPRA and the TGA claim to be taking criminal actions against non-compliant providers, the burden of proof required for such actions is higher and harder to enforce. Consequently, it is often the doctors and nurses who bear the brunt of these regulatory measures, while unscrupulous actors continue to operate with impunity.
The Musk Paradigm: Navigating the Future of Free Expression
As the debate around cosmetic surgery advertising regulations rages on, it is impossible to ignore the broader implications for free speech and the role of social media platforms like Elon Musk’s X. Musk’s defiant stance against perceived government overreach in content moderation has reignited discussions around the boundaries of free expression in the digital realm.
While safeguarding public health and consumer protection are legitimate goals, the cosmetic industry’s advertising restrictions highlight the delicate balance between these aims and upholding fundamental free speech principles. Musk’s unwavering commitment to free speech absolutism presents a stark contrast to the regulatory approach taken by Australian authorities.
As we navigate this uncharted territory, it is crucial to find a middle ground that respects the rights of businesses and individuals to engage in commercial communication while simultaneously addressing legitimate public health concerns. Musk’s disruptive influence in the social media landscape serves as a reminder that the future of free expression is inextricably linked to the evolving dynamics of technology and online discourse.
Ultimately, the path forward demands a collaborative effort between policymakers, industry stakeholders, and thought leaders. By fostering open dialogue and embracing innovative solutions, we can chart a course that upholds the principles of a free and informed society while mitigating potential risks to public safety and well-being. The cosmetic surgery advertising debate is but a microcosm of the broader challenges we face in the digital age, and it is through a nuanced and balanced approach that we can unlock the true potential of free expression while safeguarding the greater good.
Author: Dr Senthil Supramaniam MED0001193442
Registered Medical Practitioner, General Registration
Fellow(surgery) ACCSM
Editor-in-Chief: Lanna-Gerline Millien, B.Sc., MBA